Contraception - Birth Control
Individuals with debilitating MM (menstrual migraine) or MRM (menstrual related migraine) who do not have aura can be offered hormonal therapy with estrogen-progestin contraceptives to suppress their physiologic cycling of estrogen and progesterone Options for treatment:
- Continuous dosing (ie, without placebo week) of estrogen-progestin oral pills
- Continuous dosing with Nuva Ring vaginal rings (transdermal contraceptive patches are not advised for continuous dosing regimens).
- Use of estrogen-progestin oral pills with ultralow (10 to 15 micrograms) or low doses (20 to 30 micrograms).
- Use of extended-cycle preparations that reduce the number of withdrawal bleeds to four per year.
Hormonal Contraceptive Vaginal Rings – Continuous Use
NuvaRing, EluRyng (generic), Haloette (generic) (ENG/EE)
Etonogestrel and ethinyl estradiol ring (ENG/EE). There is an immediate increase in serum hormonal concentration after insertion with a slow decrease over the cycle. The concentration of EE is lower with the ENG/EE vaginal ring compared with other combined hormonal contraceptives
Common Adverse Reactions (≥ 2%): headache (including migraine) 11.2%
I can prescribe. Affordable Care Act (Obamacare) -- most insurance plans must cover all methods of birth control with little or no cost, including the ring.
Extended ring regimen up to 1 year: prior ENG/EE ring is removed and a new ring is immediately inserted every three weeks.
Common Adverse Reactions (≥ 2%): headache (including migraine) 11.2%
I can prescribe. Affordable Care Act (Obamacare) -- most insurance plans must cover all methods of birth control with little or no cost, including the ring.
Extended ring regimen up to 1 year: prior ENG/EE ring is removed and a new ring is immediately inserted every three weeks.
Annovera (SA/EE)
Segesterone and ethinyl estradiol ring — The one-year reusable segesterone acetate/ethinyl estradiol (SA/EE). The median Tmax of both hormones is approximately 2 hours after vaginal insertion; concentrations become more constant approximately 96 hours after insertion. Does not require refrigeration. Not orally active, which may appeal to lactating people who want to avoid exposing their infants to progestin. Annual. Comfortable, squishy. Available online (BlinkRx).
Common Adverse Reactions: Headache, including migraine 38.6%
Because incidence of headache in clinical trials was so high (greater than OCPs), I believe NuvaRing is a better choice.
Common Adverse Reactions: Headache, including migraine 38.6%
Because incidence of headache in clinical trials was so high (greater than OCPs), I believe NuvaRing is a better choice.
Continuous use for menstrual suppression
Data supporting continuous ring use for menstrual suppression come from studies of the ENG/EE ring. People who desire fewer days of withdrawal bleeding and are willing to tolerate some spotting can safely use an extended ring regimen whereby the prior ENG/EE ring is removed and a new ring is immediately inserted every three weeks, which omits the hormone-free week and resultant withdrawal bleeding for up to one year. Extended use is effective and does not worsen bothersome side effects, except for unscheduled bleeding. Data supporting continuous use of the SA/EE ring are not yet available, but continuous use (ie, no time break between yearly ring removal and replacement) is clinically reasonable. As with the ENG/EE ring, patients are counseled about possible increase in unscheduled bleeding.
Oral Contraceptives (OCPs)
Estrogen-containing OCPs contraindicated in migraine with aura!
For Amethyst, low dose, continuous, non-cyclic combination oral contraceptive see here.
Patches not recommended for migraine in UpToDate (use continuous vaginal ring)
Xulane Birth Control Patch (transdermal system)
- norelgestromin and ethinyl estradiol patch (Mylan Pharmaceuticals Inc.)
- (Generic of Ortho Evra, which has been discontinued.)
- Transdermal system: 150 mcg/day norelgestromin and 35 mcg/day ethinyl estradiol
- Xulane uses a 28-day (4-week) cycle. Apply a new patch to the upper outer arm, abdomen, buttock or back each week for 3 weeks (21 total days). Week 4 is patch-free. Apply each new patch on the same day of the week. Wear only one patch at a time.
- Contraindicated: headaches with focal neurological symptoms or have migraine headaches with aura; women over age 35 with any migraine headaches
- Pregnancy rate in women aged 18 to 35 years was 1.07 (95% CI: 0.60-1.76) per 100 woman-years
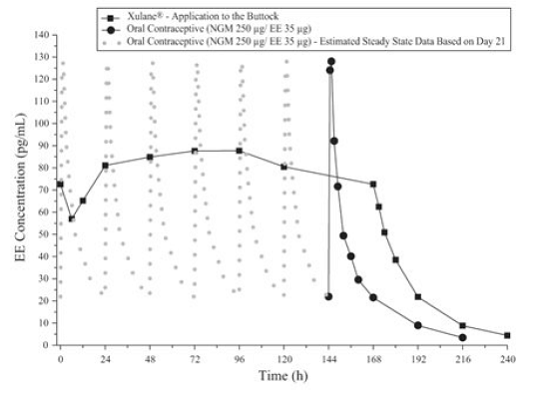
Mean Serum Concentration-Time Profiles of EE Following Once-Daily Administration of an Oral Contraceptive for Two Cycles or Application of Norelgestromin and Ethinyl Estradiol Trans dermal System for Two Cycles to the Buttock in Healthy Female Volunteers . [Oral contraceptive: Cycle 2, Days 15 to 21, Norelgestromin and Ethinyl Estradiol Transdermal System: Cycle 2, Week 3] 168 hours = 7 days
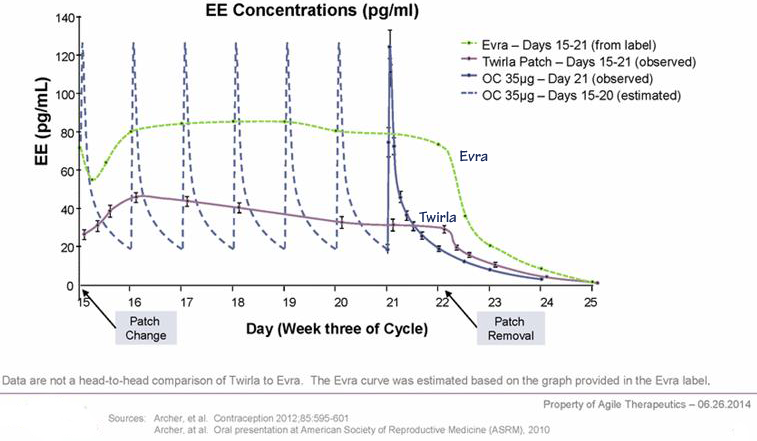
Twirla (levonorgestrel & ethinyl estradiol) transdermal system
Low dose; higher risk of pregnancy than Xulane!
- Second transdermal contraceptive patch available in the U.S.
- Daily hormone exposure is similar to that with oral contraceptives containing 30 mcg of ethinyl estradiol and 120 mcg of levonorgestrel
- Low dose; not clear if this provides reduced risk venous thromboembolism (VTE), which is one of the most significant safety concerns associated with combined hormonal contraceptive use
- Pearl Index (PI) measures the pregnancy rate per 100 women-years of drug exposure. Twirla's PI was 5.83, which exceeds Xulane. Obese women (BMI ≥30kg/m2) = 8.64; non-obese women 4.34.
- Contraindicated in women with a BMI >30 kg/m2.
- Contraindicated in women who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura.
Ortho Evra was discontinued in the U.S. after the FDA approved Xulane, a generic hormonal birth control patch, in 2014. Xulane delivers the same high levels of estrogen as the Ortho Evra patch.
Stroke Risk
- Risk of ischemic stroke in women with migraine is about 2 times higher than that of women without migraine, driven
largely by increased risk in the subgroup of women who have migraine with aura. - An association between migraine and an approximately 1.5 times increased risk of hemorrhagic stroke has also been shown.
- Greatest increase in stroke risk is seen in women with migraine in their reproductive years, who are under the age of 45, and who otherwise have few stroke risk factors. The elevation in risk of stroke is amplified by other risk factors, particularly smoking.
- Combined hormonal contraceptives with low estrogen probably have reduced stroke risk (low estrogen OCPs: 20-25 µg ethinyl estradiol; average 35 µg; some as low as 10 µg)
- Absolute risk of stroke in reproductive-aged women is low (3.56 per 100,000 women of reproductive age per year)
Relative increased ischemic stroke risk about 3-fold
- 20 µg EE 1.6 (not clear that risk is increased)
- 30-49 µg EE 2.0 (no clear that risk is increased
- >49 µg EE 2.4 (clear increased stroke risk)
One study differentiated risk by presence or absence of migraine aura and found an increased risk in the migraine with aura population (OR 6.1 in migraine with aura vs 1.8 migraine without aura group).
Progestin-only Pill (POP) [Slynd] Oral Contraceptive
Slynd®, for pregnancy prevention, is the first and only progestin-only pill (POP)
24 active pills and 4 placebo pills each cycle — 24 + 4 dosing (regular bleeding compared with continuous progestin)
Estrogen free; can be used in smoker.
Each pack includes 24 tablets containing 4 mg of drospirenone and 4 inert tablets
24 active pills and 4 placebo pills each cycle — 24 + 4 dosing (regular bleeding compared with continuous progestin)
Estrogen free; can be used in smoker.
Each pack includes 24 tablets containing 4 mg of drospirenone and 4 inert tablets
- Progestin-only pill (POP)
- High drospirenone, should be more effective than older progestin-only contraceptives
- Appropriate when the use of combination methods should be avoided due to elevated cardiovascular risks, including smokers age 35 and older and women with hypertension, migraines with aura, multiple cardiovascular risk factors, or a history of thrombosis.