Menopausal Vasomotor Symptoms
Antidepressants produced modest improvements in vasomotor symptoms.
Venlafaxine and low-dose oral estradiol equally effective in reducing hot flashes (50% vs 30% with placebo)
Reed SD, LaCroix AZ, Anderson GL, Ensrud KE, Caan B, Carpenter JS, Cohen L, Diem SJ, Freeman EW, Joffe H, Larson JC, McCurry SM, Mitchell CM, Newton KM, Sternfeld B, Guthrie KA. Lights on MsFLASH: a review of contributions. Menopause. 2020 Apr;27(4):473-484.
Reed SD, LaCroix AZ, Anderson GL, Ensrud KE, Caan B, Carpenter JS, Cohen L, Diem SJ, Freeman EW, Joffe H, Larson JC, McCurry SM, Mitchell CM, Newton KM, Sternfeld B, Guthrie KA. Lights on MsFLASH: a review of contributions. Menopause. 2020 Apr;27(4):473-484.
Veozah (fezolinetant) - NK3 Receptor Antagonist
Dose: 45 mg q day
The neurokinin 3 (NK3) receptor antagonist fezolinetant (Veozah) is FDA-approved for treatment of moderate to severe VMS. Blocking neurokinin B activity restores hypothalamic thermoregulatory control, reducing VMS. In clinical trials in women with moderate to severe VMS associated with menopause, reductions in the frequency and severity of VMS were statistically significantly greater with fezolinetant than with placebo.19,20 Fezolinetant can cause abdominal pain, diarrhea, insomnia, back pain, hot flushes, and hepatic transaminase elevations. Fezolinetant is contraindicated for use with a CYP1A2 inhibitor.
The neurokinin 3 (NK3) receptor antagonist fezolinetant (Veozah) is FDA-approved for treatment of moderate to severe VMS. Blocking neurokinin B activity restores hypothalamic thermoregulatory control, reducing VMS. In clinical trials in women with moderate to severe VMS associated with menopause, reductions in the frequency and severity of VMS were statistically significantly greater with fezolinetant than with placebo.19,20 Fezolinetant can cause abdominal pain, diarrhea, insomnia, back pain, hot flushes, and hepatic transaminase elevations. Fezolinetant is contraindicated for use with a CYP1A2 inhibitor.
Transdermal Estrogen/Progestin Combinations
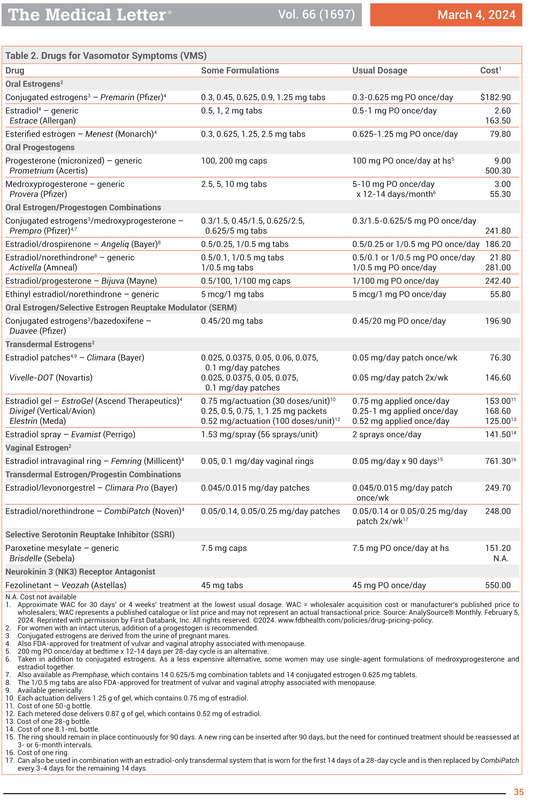
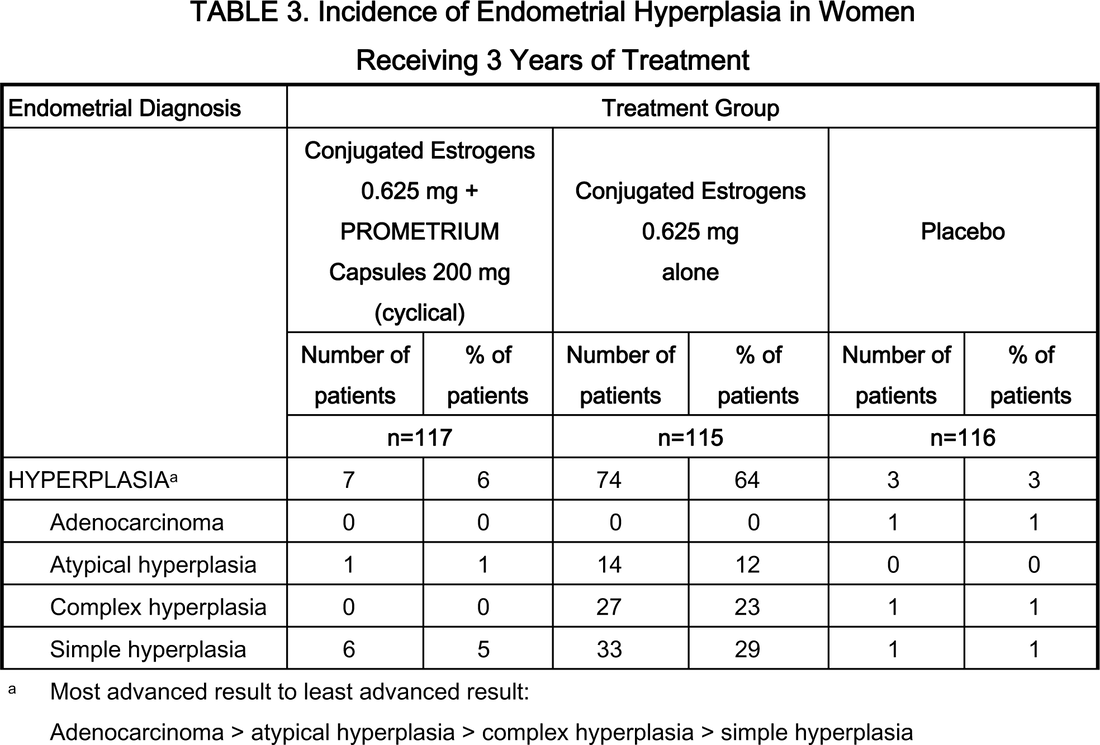
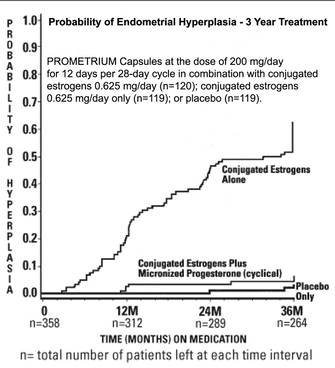
For menopausal vasomotor symptoms. Women with an intact uterus who take a systemic estrogen should also take a progestogen to reduce the risk of endometrial hyperplasia and adenocarcinoma. Progestin should not be used in women who have had uterus removed. Transdermal formulations are as effective as oral estrogens in reducing vasomotor symptoms and may be safer.
Climara Pro – Estradiol/levonorgestrel
Estradiol 0.045 mg + levonorgestrel 0.015 mg; per day; transdermal system.
Once per week.
CombiPatch – Estradiol/norethindrone
Estradiol 0.05 mg + norethindrone acetate 0.14 mg; per day; transdermal system.
Estradiol 0.05 mg + norethindrone acetate 0.25 mg; per day; transdermal system.
Twice per week.
Climara Pro and CombiPatch are in table below. Note that Climara Pro is once a week.
Climara Pro – Estradiol/levonorgestrel
Estradiol 0.045 mg + levonorgestrel 0.015 mg; per day; transdermal system.
Once per week.
CombiPatch – Estradiol/norethindrone
Estradiol 0.05 mg + norethindrone acetate 0.14 mg; per day; transdermal system.
Estradiol 0.05 mg + norethindrone acetate 0.25 mg; per day; transdermal system.
Twice per week.
Climara Pro and CombiPatch are in table below. Note that Climara Pro is once a week.